Sugar is a carbohydrate compound that is composed of 12 carbon atoms. The number of bonds whether single double or triple is determined by the number of shared.
Covalent Bond Definition Types And Examples
These electron pairs are termed shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is termed covalent bonding.
. In the F 2 molecule the FF latex sigmalatex covalent bond is formed by the overlap of p z orbitals of the two F atoms each containing an unpaired electron. Thus the valence theory doesnt explain the covalent bond of the methane molecule. The bond dipoles are colored.
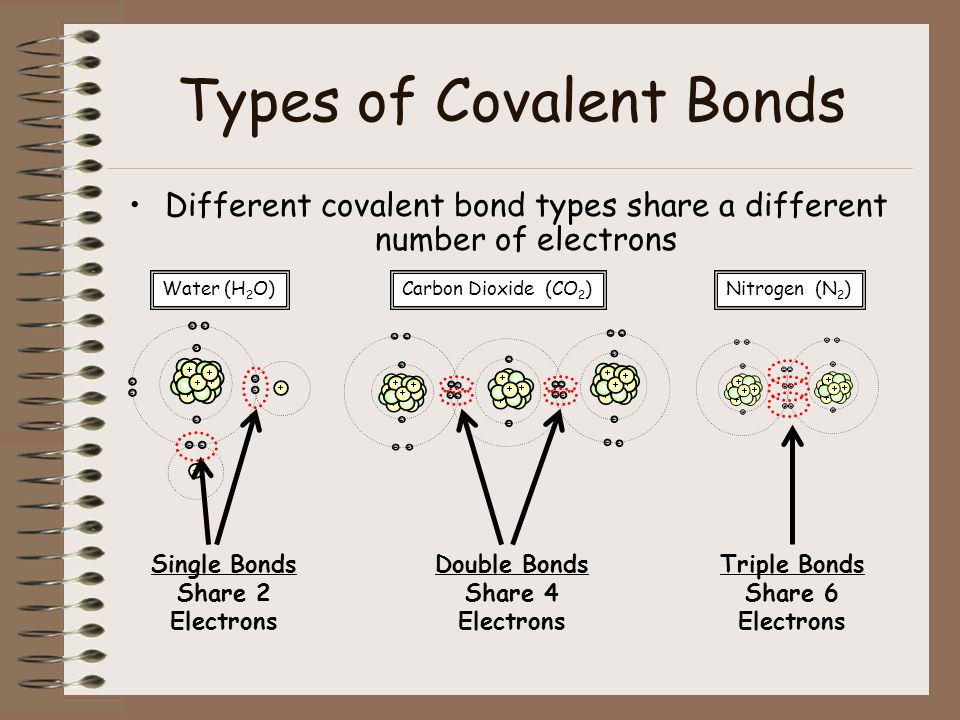
Lets look at three different types of covalent bonds. Since the nature of the overlapping orbitals is different in H 2 and F 2 molecules bond strength and bond. This interactive activity from ChemThink describes covalent bondinga type of chemical bond that involves the sharing of electrons.
The resulting material is the daughter isotope. This theory is used to explain the covalent bond formation in many molecules. Stable isotopes either never decay or else decay very slowly.
There are different types of hybridization-based on the mixing of the orbitals. Radioactive isotopes undergo decay. The number of protons for different isotopes of an element does not change.
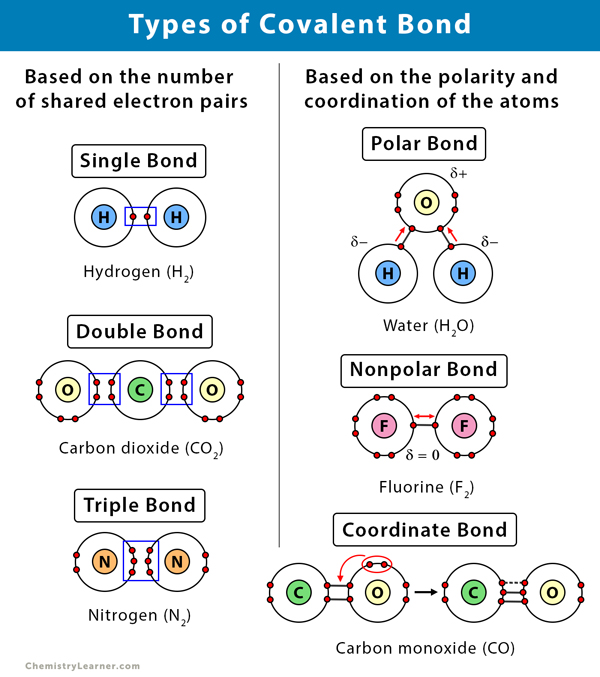
Single bonds are a type of covalent bond formed from the sharing of two electrons between two atoms one electron. The negative end of one molecule attracts the positive end of. In a molecule when a hydrogen atom is linked to a highly electronegative atom it attracts the shared pair of electrons more and so this end of the molecules becomes slightly negative while the other end becomes slightly positive.
Investigate the attractive and repulsive forces that act on atomic particles and how the sharing of electrons can keep atoms together. Notice that the area of overlap always occurs between the nuclei of the two bonded atoms. The following diagram shows four possible orientations of the O-H bonds.
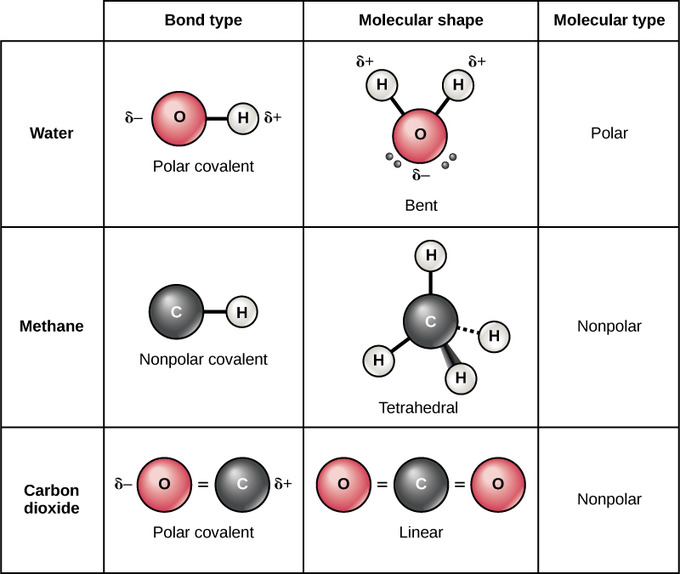
When an isotope decays the starting material is the parent isotope. A type of chemical. For example if we talk about water H2O it is a polar covalent bond.
These are all possible overlaps between different types of atomic orbitals that result in the formation of a sigma bond between two atoms. An ionic bond is a type of chemical bond in which the atoms have different electronegativity values from each other. Give some specific examples.
Single covalent bonds occur when one pair of electrons is shared between atoms as part of a molecule or. The polar covalent character of water allows its molecules to become attracted to many other different types of molecules and disrupt their pre-existing bonds. Explain the difference using the octet rule.
Bond characteristics vary depending on a variety of factors such as molecular nature solid type crystalline or amorphous and so on. In a covalent compound it is the sum of their covalent radii. What are some differences between an ionic bond and a covalent bond.
On the other hand a polar covalent bond is a covalent bond in which the shared electrons are more attracted to one of the atoms than the other. Since there are two O-H bonds in water their bond dipoles will interact and may result in a molecular dipole which can be measured. A non-polar covalent bond is a bond where the shared electrons are shared equally.
Not all isotopes are radioactive. For a covalent molecule AB the bond length is given by d r a r b. What are the Conditions for Hydrogen Bonding.
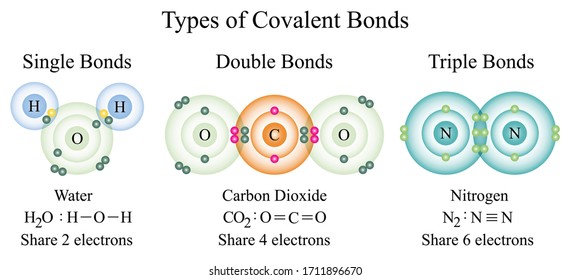
Two or more electrons are shared to form covalent bonds. A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. The Three Covalent Bonds.
The shred electrons are more likely to be near the atom whose electronegativity is higher. The hybridization concept explains the formation of identical 4 C-H bonds and the tetrahedral shape of the molecule. There are many different types of bonds including covalent ionic and van der Waals bonds.
Interactive problems to aid students of organic chemistry. Learn about trends in the. It is determined experimentally by x-ray diffraction or electron diffraction method or spectroscopic method.
There are 250 isotopes of the 90 naturally occurring. The bond length in chemical bonding is the sum of their ionic radii in an ionic compound. For example sodium Na and chlorine Cl form an ionic bond to make NaCl table salt.
According to this concept when a carbon atom reacts with a hydrogen atom the electrons in. The practice problems offered here are chiefly interactive and should provide a useful assessment of understanding at various stages in the development of the subject. A chemical bond.
Examples of Covalent Bond. In the case of water we know that the O-H covalent bond is polar due to the different electronegativities of hydrogen and oxygen. However it is important to note that the bond formed between two individual water molecules is a hydrogen bond and not covalent.
An example is water. See how two hydrogen atoms interact with each other to create a covalent bond. Sodium and fluorine undergoing a redox reaction to form sodium fluoride.
However in a covalent bond the atoms are bound to share electrons.
2 1i Covalent Bonds And Other Bonds And Interactions Biology Libretexts

Notes 5 3 Covalent Bonds Covalent Bond A Force That Bonds Two Atoms Together By A Sharing Of Electrons Each Pair Of Shared Electrons Creates Ppt Download
0 Comments